Direct cross-coupling between alkenes and tetrahydrofuran with a platinum-loaded titanium oxide photocatalyst†
Abstract
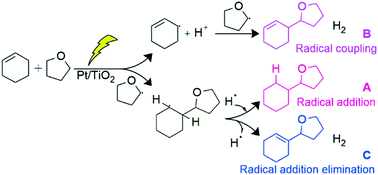
A Pt-loaded TiO2 photocatalyst successfully catalyzed the direct cross-coupling between various alkenes and tetrahydrofuran (THF) without any additional oxidizing agent. The reaction between cyclohexene and THF gave three cross-coupling products, namely, 2-cyclohexyltetrahydrofuran (A), 2-(cyclohex-2-en-1-yl)tetrahydrofuran (B) and 2-(cyclohex-1-en-1-yl)tetrahydrofuran (C), along with gaseous hydrogen. The mechanistic study revealed that these products were formed through different individual mechanisms: successive addition of two radical species, a 2-tetrahydrofuranyl radical and a hydrogen radical, to the double bond of cyclohexene for A, coupling of a 3-cyclohexenyl radical and a 2-tetrahydrofuranyl radical for B, and 2-tetrahydrofuranyl radical addition and hydrogen radical elimination at the double bond of cyclohexene for C. Among these three mechanisms, those for B and C are dehydrogenative. In this photocatalytic reaction system, since the cyclohexene molecule has enough reactivity, due to the localized π electron density, the Pt nanoparticles loaded on the TiO2 function not as a metal catalyst but as an electron receiver to enhance the charge separation, although the dehydrogenative cross-coupling of benzene with THF requires Pd metal catalysis.


 Please wait while we load your content...
Please wait while we load your content...