Charge behavior in a plasmonic photocatalyst composed of Au and TiO2†
Abstract
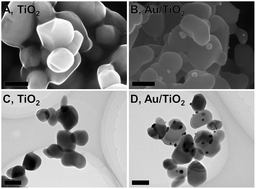
Photocatalysts are widely used in environmental and energy fields because of their sustainable characteristics. Recently, a plasmonic photocatalyst which can absorb more photons has been invented in order to enhance the photocatalytic activity of conventional photocatalysts (such as TiO2). However, the reported activities are not as high as expected. One of the reasons for this is that the mechanism of photocatalysis is not very well understood yet. Thus, here, redox reaction sites on a Au nanoparticle-deposited TiO2 (Au/TiO2) plasmonic photocatalyst are visualized using a chemical microanalytical technique for investigating charge behaviors in Au/TiO2. Ag and Mn oxide are deposited on the surface of Au/TiO2 by reduction and oxidation reactions, respectively. The amounts of Ag and Mn oxide deposited on different sites of Au/TiO2 are measured to clarify the percentage of the consumption of generated charges at each site. A probable charge behavior is proposed based on literature reports and the results obtained in this work.


 Please wait while we load your content...
Please wait while we load your content...