Polyoxometalates covalently combined with graphitic carbon nitride for photocatalytic hydrogen peroxide production†
Abstract
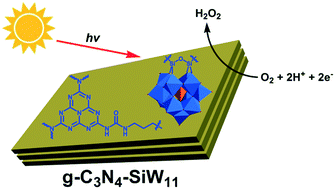
The polyoxometalate (POM) cluster [SiW11O39]8− (SiW11) with photoreductive ability has been successfully covalently combined with graphitic carbon nitride (g-C3N4) through an organic linker strategy. The hybrid catalyst g-C3N4–SiW11 exhibits efficient catalytic performance (15.2 μmol h−1) for photocatalytic H2O2 production in the presence of methanol and can stabilize the formed H2O2 under sunlight irradiation (AM 1.5 filter). The Koutecky–Levich plot obtained from electrochemical rotating disk electrode (RDE) analysis of the oxygen reduction reaction (ORR) for g-C3N4–SiW11 reveals that the value of electron transfer during the ORR process is 2.76. Combining the electron spin resonance (ESR), Koutecky–Levich plots, O2 temperature programmed desorption (O2-TPD) and density functional theory (DFT) calculation results, the enhanced O2 adsorption of g-C3N4–SiW11 can promote the two-electron reduction of O2 to H2O2.


 Please wait while we load your content...
Please wait while we load your content...