Mechanism of supported Ru3Sn7 nanocluster-catalyzed selective hydrogenation of coconut oil to fatty alcohols†
Abstract
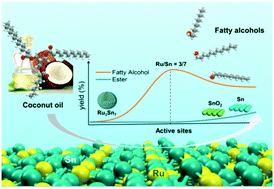
As a promising hydrotreating catalyst, it was previously reported that Ru⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Sn (Ru electronically interacts with Sn oxides) on RuSn/SiO2 was the active site for fatty acid hydrogenation, but here in this work we found that Ru3Sn7 nanoclusters on RuSn/SiO2 were responsible for the selective hydrogenation of diverse fatty acids and coconut oil to fatty alcohols. The XPS results indicated no interaction between Snδ+ and Ru0, suggesting that SnOx may exist as isolated species. In contrast, the binding energy shifts of Ru0 and Sn0 in the XPS spectra demonstrated a strong interaction, as a result of the formation of Ru3Sn7 alloy nanoclusters. It was demonstrated that the highest yield of fatty alcohol was obtained with a Sn/Ru ratio of 7/3 (hydrogenation rate: 2.45 g g−1 h−1), and the careful selection of the Sn/Ru ratio and reduction temperatures greatly suppressed the formation of Sn and SnO2 phases. The ratio of the stearyl alcohol formation rate to its consumption rate was 40.8 with Ru3Sn7/SiO2 under the selected conditions. In catalysts with a Sn/Ru ratio higher than 7/3, the presence of additional SnO2 catalyzes the formation of undesired esters at a rate of 0.31 g g−1 h−1. Excess SnO2 would be reduced to Sn at temperatures higher than 600 °C, while Sn can catalyze ester by-product formation at a rate of 0.88 g g−1 h−1. The DFT calculations showed that CH3COOH adsorbs on the Ru3Sn7 (111) surface via Sn–O interactions at the two top sites of adjacent surface Sn atoms, and such adsorption was mainly due to the electrostatic interactions between the molecule and the positively charged surface Sn atoms. The charge density difference (CDD) plots of co-adsorbed CH3CO* and OH* intermediates indicated the bonding relationships between Sn–O and Ru–α-C, suggesting that the surface Sn atoms also took part in the catalytic reaction as an important surface sorption site as well as a Ru3Sn7 structure component, while Ru atoms bonded with α-C and hydrogenated the adsorbed intermediate species with the adsorbed H* to the final alcohol. A further indication that Ru3Sn7 was the active species in the bimetallic Ru–Sn catalyst was given by the much lower energy barrier for hydrogenation of acetic acid in Ru3Sn7 (111) (81.0 kJ mol−1) compared to Ru (0001) (123.5 kJ mol−1).
Sn (Ru electronically interacts with Sn oxides) on RuSn/SiO2 was the active site for fatty acid hydrogenation, but here in this work we found that Ru3Sn7 nanoclusters on RuSn/SiO2 were responsible for the selective hydrogenation of diverse fatty acids and coconut oil to fatty alcohols. The XPS results indicated no interaction between Snδ+ and Ru0, suggesting that SnOx may exist as isolated species. In contrast, the binding energy shifts of Ru0 and Sn0 in the XPS spectra demonstrated a strong interaction, as a result of the formation of Ru3Sn7 alloy nanoclusters. It was demonstrated that the highest yield of fatty alcohol was obtained with a Sn/Ru ratio of 7/3 (hydrogenation rate: 2.45 g g−1 h−1), and the careful selection of the Sn/Ru ratio and reduction temperatures greatly suppressed the formation of Sn and SnO2 phases. The ratio of the stearyl alcohol formation rate to its consumption rate was 40.8 with Ru3Sn7/SiO2 under the selected conditions. In catalysts with a Sn/Ru ratio higher than 7/3, the presence of additional SnO2 catalyzes the formation of undesired esters at a rate of 0.31 g g−1 h−1. Excess SnO2 would be reduced to Sn at temperatures higher than 600 °C, while Sn can catalyze ester by-product formation at a rate of 0.88 g g−1 h−1. The DFT calculations showed that CH3COOH adsorbs on the Ru3Sn7 (111) surface via Sn–O interactions at the two top sites of adjacent surface Sn atoms, and such adsorption was mainly due to the electrostatic interactions between the molecule and the positively charged surface Sn atoms. The charge density difference (CDD) plots of co-adsorbed CH3CO* and OH* intermediates indicated the bonding relationships between Sn–O and Ru–α-C, suggesting that the surface Sn atoms also took part in the catalytic reaction as an important surface sorption site as well as a Ru3Sn7 structure component, while Ru atoms bonded with α-C and hydrogenated the adsorbed intermediate species with the adsorbed H* to the final alcohol. A further indication that Ru3Sn7 was the active species in the bimetallic Ru–Sn catalyst was given by the much lower energy barrier for hydrogenation of acetic acid in Ru3Sn7 (111) (81.0 kJ mol−1) compared to Ru (0001) (123.5 kJ mol−1).


 Please wait while we load your content...
Please wait while we load your content...