A DFT study of chlorine coverage over late transition metals and its implication on 1,2-dichloroethane hydrodechlorination†
Abstract
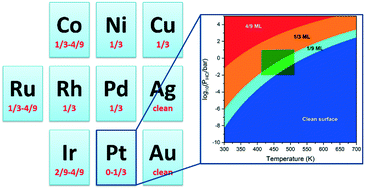
Atomic chlorine is known to interact strongly with most late transition metal surfaces. This work aims to identify the relevant chlorine coverage under typical 1,2-dichloroethane hydrodechlorination conditions (T = 423–523 K, PHCl = 10−2–101 bar) and its impact on the reaction energetics. Density functional theory calculations (GGA-PW91) were performed to study the adsorption of chlorine up to 1 monolayer over the close-packed facets of 10 late transition metals (Pt, Co, Ni, Cu, Ru, Rh, Pd, Ag, Ir, Au). The ab initio phase diagram of each chlorine/metal system was constructed. The phase diagrams predicted approximately 1/3 ML chlorine coverage on all metals other than Ag and Au, which remain clean surfaces under hydrodechlorination conditions. The presence of surface chlorine was also found to significantly destabilize the adsorption of reaction intermediates, thus shifting the reaction potential energy diagram upwards. Our results emphasize the importance of explicitly including adsorbed chlorine spectators in properly modeling the catalytic surfaces for the 1,2-dichloroethane hydrodechlorination reaction.


 Please wait while we load your content...
Please wait while we load your content...