Supported Fe/MnOx catalyst with Ag doping for remarkably enhanced catalytic activity in Fischer–Tropsch synthesis†
Abstract
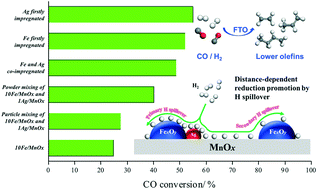
Although the Mn promoter has been widely demonstrated to be beneficial to promoting the adsorption and dissociation of CO and therefore increasing the selectivity to light olefins in Fe-based FTS, it also brings about a negative effect on the catalytic activity due to its strong interaction with Fe oxides. In this study, Fe oxide was directly dispersed on a synthesized mesoporous spherical-like MnOx support and further doped with Ag nanoparticles to investigate its impact on the FTS reaction. XRD and H2-TPR characterization confirmed that Mn and Fe tend to form an FeMn solid solution over a 10 wt% Fe/MnOx catalyst. As expected, a small amount of Ag doping on it could remarkably increase the catalytic activity by 1–5 times and even the selectivity to light olefins; this could be attributed to easier reduction of the Fe oxide, Mn oxide and FeMn solid solution due to the hydrogen (H) spillover generated by Ag as the reduced metallic Fe favors the formation of active Fe carbide and more O vacancies in MnOx facilitate CO adsorption and the rapid removal of dissociated O atoms on Fe carbide. Furthermore, the FTS activity related to the H spillover was intensively investigated over the MnOx-supported catalysts with different impregnation sequences of Ag and Fe, and over the hybrid 10Fe/MnOx and 1Ag/MnOx catalysts with particle and powder mixing in order to regulate the distance between Fe and Ag. It was found that the primary and secondary ways of H spillover are involved in the promoted reduction process, which was illustrated by XRD, H2-TPR, H2-TPD and CO-TPD characterization combined with catalytic results. The secondary spillover exhibited a much milder effect on the reduction promotion with a farther distance of Fe and Ag. Compared with the co-impregnated 1Ag10Fe/MnOx catalyst, the sequential impregnation of Ag and Fe onto the MnOx support, named the 10Fe/1Ag/MnOx catalyst, showed higher catalytic activity, which might be due to less formation of FeMn solid solution resulting from firstly introduced Ag on MnOx. Interestingly, both Ag-doped and Ag-free 10Fe/MnOx catalysts showed a rapid increase in CO conversion after several tens of hours of stable reaction and the CO conversion can reach 80–90% after a 100 hour test, and the possible reason for this was discussed based on HAADF-STEM observations of spent catalyst samples.


 Please wait while we load your content...
Please wait while we load your content...