Cinnamaldehyde hydrogenation using Au–Pd catalysts prepared by sol immobilisation†
Abstract
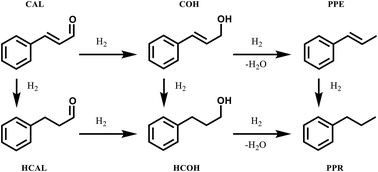
We report the catalytic performance of Au–Pd nanoparticles prepared via a sol immobilisation technique for the catalytic hydrogenation of cinnamaldehyde under mild reaction conditions. We synthesised a series of bimetallic Au–Pd colloidal supported nanoparticles with different Au : Pd molar ratios and optimized the experimental parameters to achieve the best catalyst performance. The optimum catalytic activity for the hydrogenation of cinnamaldehyde was observed for Au50Pd50/TiO2 (with a Au : Pd molar ratio of 1 : 1), while the monometallic Pd/TiO2 was the most selective towards hydrocinnamaldehyde. The catalysts have been structurally characterised and FTIR analysis showed that the presence of adsorbed carbonyl surface species in used catalyst materials is coupled with Pd leaching, which is the main reason for catalyst deactivation. The effect of calcination on the most active Au–Pd/TiO2 was studied in the range 110–400 °C and a direct correlation between the rise in calcination temperature and catalyst stability and selectivity was observed. These results emphasise the importance of tuning the Au–Pd molar ratio and understanding the metal–support interaction of catalysts synthesised for hydrogenation reactions, such as cinnamaldehyde hydrogenation.


 Please wait while we load your content...
Please wait while we load your content...