Spatiotemporal coke formation over zeolite ZSM-5 during the methanol-to-olefins process as studied with operando UV-vis spectroscopy: a comparison between H-ZSM-5 and Mg-ZSM-5†
Abstract
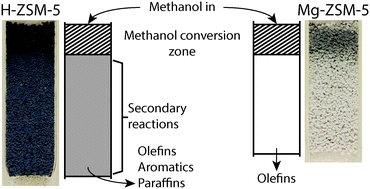
In this work, during the methanol-to-olefins (MTO) reaction, the formation of hydrocarbon pool species as well as the accumulation of coke and coke precursor molecules were monitored with operando UV-vis spectroscopy. Using three UV-vis probes at different positions along the reactor bed simultaneously, the formation of active species and coke along the reactor bed was measured. Two catalyst materials have been compared with this operando approach: H-ZSM-5 with a Si/Al ratio of 50; and the same zeolite material that was modified using magnesium. It was revealed using spatiotemporal UV-vis spectroscopy that for both H-ZSM-5 and Mg-ZSM-5 a coke front is formed in the beginning of the reactor bed, and this coke front travels through the catalyst bed. Once the coke front reaches the end of the bed, deactivation of the catalyst material is observed. The magnesium modification resulted in extended lifetime of the catalyst, as well as higher selectivity towards olefins compared to H-ZSM-5. Operando UV-vis spectroscopy data revealed that the increase in lifetime of the catalyst was accompanied by a slower progression of the coke front through the catalyst bed, and less formation of aromatic species, especially in the parts of the catalyst bed behind the methanol conversion zone, i.e., behind the coke front. Additional experiments where MTO products, i.e., ethylene and propylene, were fed to the reactor, showed that the formation of aromatic species behind the methanol conversion zone on H-ZSM-5 are the result of aromatization of the products of methanol conversion, such as ethylene and propylene. On Mg-ZSM-5, however, ethylene and propylene were less reactive, resulting in less aromatics formation and a higher selectivity towards olefins. Based on these results, a distinction was made between primary coke, i.e., coke that forms due to the conversion of methanol into hydrocarbons, and secondary coke, i.e. coke that is formed when the MTO products, such as propylene and ethylene, undergo subsequent aromatization further in the reactor bed. The reason for the observed differences between H-ZSM-5 and Mg-ZSM-5 is that the Mg-modification results in a decrease in the number of Brønsted acid sites, as well as the creation of Lewis acid sites. The decrease in Brønsted acid sites limits the formation of secondary coke that is caused by olefin aromatization. This in turn leads to the increased lifetime and higher observed olefin selectivity of Mg-ZSM-5 relative to H-ZSM-5.


 Please wait while we load your content...
Please wait while we load your content...