Merrifield resin-assisted routes to second-generation catalysts for olefin metathesis†
Abstract
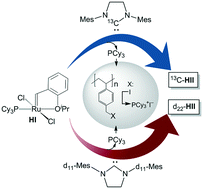
Phosphine-scavenging resins can significantly facilitate the synthesis of highly active Ru metathesis catalysts, including the second-generation Grubbs, Hoveyda, and indenylidene catalysts (GII, HII, InII). These catalysts are customarily prepared by ligand exchange of the corresponding first-generation catalysts with the N-heterocyclic carbene (NHC) H2IMes. The PCy3 coproduct is conventionally removed by pentane extraction, but the partial solubility of the desired Ru products can cause product losses of over 20%. Sequestration of the PCy3 coproduct with CuCl is more efficient, but is undesirable given the potential for non-innocent copper residues. Use of the arylsulfonic acid resin Amberlyst-15 delivers near-quantitative catalyst yields, but the high acidity of the resin leads to problems with reproducibility and decomposition. An alternative approach is described, in which a neutral Merrifield resin (crosslinked polystyrene with pendant p-C6H4CH2I groups; MF-I) is used to sequester PCy3 as the covalently-tethered benzylphosphonium salt. Addition of MF-I following complete ligand exchange effects quantitative uptake of free PCy3 (and any residual free NHC) within 45 min at RT: the clean products are isolated by filtration, in ca. 95% yield. These yields compare well with those obtained via the Amberlyst-15 route, without the challenges due to resin acidity. The efficacy of this methodology is demonstrated in the synthesis of isotopically-labelled derivatives of HII, in which the H2IMes ligand bears a 13C-label at the carbene carbon, or perdeuterated mesityl rings.
- This article is part of the themed collection: Catalysis Science & Technology 10th Anniversary Symposium


 Please wait while we load your content...
Please wait while we load your content...