Citric acid-assisted hydrothermal synthesis of a self-modified Bi2SiO5/Bi12SiO20 heterojunction for efficient photocatalytic degradation of aqueous pollutants
Abstract
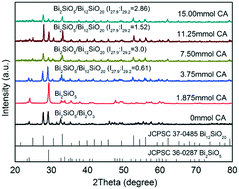
A self-modified Bi2SiO5/Bi12SiO20 heterojunction was synthesized for the first time via a citric acid-assisted hydrothermal method. It was found that citric acid (CA) played two roles in the formation of the Bi2SiO5/Bi12SiO20 heterojunction: enhancement of the specific surface area due to the release of many volatiles from the decomposition of CA and tuning the weight ratio of Bi2SiO5 and Bi12SiO20 in the heterojunction by forming different complexes with Bi3+. Thus, a self-modified Bi2SiO5/Bi12SiO20 heterojunction prepared at a CA dosage of 11.25 mmol presented an I27.9 : I29.2 value of 1.52 and a BET specific surface area of 32.6 m2 g−1, 5.7 times that without addition of CA. It showed the highest first-order apparent reaction constant of 0.1694 min−1 in the photocatalytic degradation of acid orange 7 (AO7), which is 1.8, 53.0, and 2.2 times that of Bi2SiO5, Bi12SiO20, and TiO2 (P25), respectively. The capture experiments show that the main active species that degraded AO7 are not O2˙− and ˙OH but h+vb. During the AO7 degradation, four intermediates, sulfonic acid, oxalic acid, benzenesulfonic acid, and 4-aminobenzenesulfonic acid, were identified by capillary electrophoresis/capacitively coupled contactless conductivity detection. Finally, other pollutants, rhodamine B, p-chlorophenol, and tetracycline, were also found to be efficiently removed by the Bi2SiO5/Bi12SiO20-mediated photocatalysis system.


 Please wait while we load your content...
Please wait while we load your content...