Support effect of Mn-based catalysts for gaseous elemental mercury oxidation and adsorption
Abstract
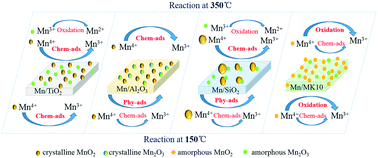
Mn-Based catalysts with a Mn loading of 4 wt% were prepared using an impregnation method. Their Hg0 removal efficiencies were in the following order: 4% Mn/MK10 (montmorillonite K 10) > 4% Mn/SiO2 > 4% Mn/TiO2 > 4% Mn/Al2O3. Results from various characterization techniques, such as BET, XRD, STEM, H2-TPR, Hg0-TPD and XPS, suggested that the species, morphologies, and distributions of MnOx led to the different performances in Hg0 removal. The support effect on Hg0 adsorption and oxidation was studied. For 4% Mn/Al2O3 and 4% Mn/SiO2, physical adsorption dominates Hg0 removal at low temperature (150 °C), while chemical adsorption is more important at high temperature (350 °C). At both low and high temperatures, chemical adsorption and oxidation play leading roles in 4% Mn/TiO2 and 4% Mn/MK10, respectively. The effect of MnOx species and their morphologies on Hg0 oxidation was investigated. Amorphous MnO2 benefits Hg0 oxidation at both low and high temperatures, while amorphous Mn2O3 only facilitates Hg0 oxidation at high temperature. Hg0 oxidation on Mn-based catalysts follows a Mars–Maessen mechanism where the lattice oxygen of MnOx reacts with the absorbed Hg0.


 Please wait while we load your content...
Please wait while we load your content...