A highly efficient sulfonic acid resin for liquid-phase carbonylation of dimethoxymethane
Abstract
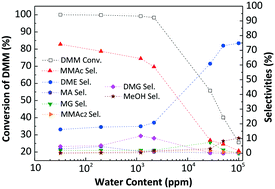
Methyl methoxyacetate (MMAc), which is an important fine chemical, can be used as an intermediate to produce ethylene glycol from syngas. For the reported vapor-phase carbonylation of dimethoxymethane (DMM), the reaction was conducted under a higher (>100) CO/DMM molar ratio with a lower CO conversion (<0.5%). This paper systematically studied the effects of different zeolites and sulfonic acid resin catalysts, reaction temperature, CO pressure, CO/DMM ratio, reaction time, drying temperature, as well as H2O and methanol contents on DMM conversion and products selectivities, using a slurry phase reactor. A highly efficient sulfonic acid resin was selected without the assistance of a solvent and the DMM conversion reached nearly 100%, with 64.39% MMAc selectivity at 110 °C and 5.0 MPa for 6 h, with a CO/DMM ratio of only 1.67/1. The effects of H2O (32 ppm–10 wt%) and methanol (0.5–10 wt%) content on the carbonylation efficiency were also systematically studied. After removing H2O from the DMM and resin catalyst, the MMAc selectivity got as high as 74.14% under the same reaction conditions. According to these reaction results and a precise GC-MS analysis, DMM2, MG, DMG, MA, and MMAc2 were evidently produced, along with MMAc, DME, MF, and methanol. We propose reaction routes from these results, and anticipate that this direct carbonylation of DMM to produce an MMAc process is promising for industrial manufacturing.


 Please wait while we load your content...
Please wait while we load your content...