DFT study of CO2 hydrogenation catalyzed by a cobalt-based system: an unexpected formate anion-assisted deprotonation mechanism†
Abstract
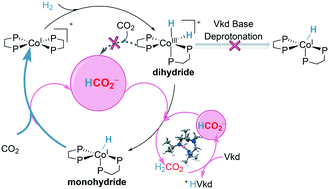
Catalyzed hydrogenation of CO2 by earth-abundant metal complexes is a promising strategy to utilize hydrogen as a kind of sustainable energy and to reduce greenhouse gases. A systematic density functional theory (DFT) study is presented for CO2 hydrogenation catalyzed by the Co(dmpe)2H (dmpe: 1,2-bis(dimethylphosphino)-ethane) complex with Verkade's base as an additive. An unexpected formate anion-assisted deprotonation mechanism is unfolded, which is different from the generally accepted additive base-catalyzed deprotonation mechanism. The complete catalytic cycle involves three main steps: (i) oxidative addition, (ii) deprotonation of the dihydride complex, and (iii) hydrogenation of CO2. The cobalt monohydride complex Co(dmpe)2H is found to be the catalytically active species and the rate-determining step is the hydrogenation of CO2 (ΔG‡ = 20.9 kcal mol−1). Furthermore, the hydride transfer process prefers the reductive elimination mechanism (ΔG‡ = 20.9 kcal mol−1) to the direct transfer mechanism (ΔG‡ = 23.3 kcal mol−1). In contrast, the cobalt dihydride complex [Co(dmpe)2H2]+ is less likely to be the active species, which should be deprotonated to the cobalt monohydride species for further hydrogenation. The deprotonation of cobalt dihydride to cobalt monohydride is found to be promoted by the formate anion (ΔG‡ = 10.5 kcal mol−1) instead of Verkade's base (ΔG‡ = 36.9 kcal mol−1). On the other hand, a direct heterolytic cleavage of H2 assisted by the base to cobalt monohydride is less feasible compared with the oxidative addition of H2. These results can well explain the necessity for oxidative addition and the appearance of formic acid after Verkade's base has run out in the experiments, and are in good agreement with the experimental observation that the hydrogenation of CO2 instead of the deprotonation is the rate-determining step. The present results provide sharp insights and helpful guidelines for designing novel hydrogenation systems with transition metal complexes and bases.


 Please wait while we load your content...
Please wait while we load your content...