Role of lattice oxygen and oxygen vacancy sites in platinum group metal catalysts supported on Sr3Fe2O7−δ for NO-selective reduction†
Abstract
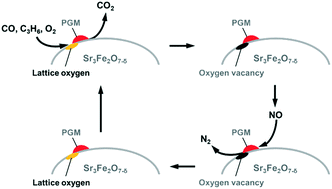
This study demonstrates that NO-selective reduction with C3H6 and CO proceeds over platinum group metal (PGM; Pd, Rh, or Pt) catalysts supported on Sr3Fe2O7−δ with a layered perovskite-type oxide. Among the examined catalysts, Pd-loaded Sr3Fe2O7−δ having high oxygen storage capacity shows the highest catalytic activity at stoichiometric oxygen concentration because the Pd catalyst can release the lattice oxygen of Sr3Fe2O7−δ at lower temperature compared to the Rh- or Pt-loaded catalyst. When the NO-selective reduction at 773 K is carried out at various oxygen concentrations, PGM/Sr3Fe2O7−δ shows superior catalytic activity over a wide range of oxygen concentrations to PGM/Al2O3, which does not have oxygen storage capacity. The oxygen vacancy sites in Sr3Fe2O7−δ, which are generated by the oxidation of C3H6 and CO over PGM/Sr3Fe2O7−δ, are revealed to receive oxygen ions formed by NO reduction on the PGM species; as a result, the PGM species maintain their metal state and act as active sites for NO reduction. Namely, Sr3Fe2O7−δ plays the role of an “oxygen buffer” in inhibiting the PGM-to-oxide transformation. Pt-loaded Sr3Fe2O7−δ can utilize the oxygen vacancy sites more effectively for the catalytic reaction than the Pd and Rh catalysts. To the best of our knowledge, this is the first report on the effective application of perovskite materials with oxygen storage capacity as catalyst supports for NO-selective reduction. The study results are expected to provide valuable information for designing a novel catalyst based on oxygen storage materials.


 Please wait while we load your content...
Please wait while we load your content...