Hydrogen transfer reactions relevant to Guerbet coupling of alcohols over hydroxyapatite and magnesium oxide catalysts
Abstract
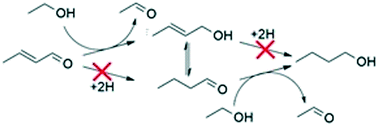
Hydrogenation and dehydrogenation reactions were performed over hydroxyapatite (Ca10(PO4)6(OH)2, HAP) and magnesia (MgO) to explore their role in the reaction network for the Guerbet coupling of ethanol to butanol. In particular, the dehydrogenation of benzyl alcohol at 633 K and the hydrogenation of ethene and acetone at 473 K using both H2 and ethanol as a hydrogen source were studied. The H2–D2 exchange reaction at room temperature and the Guerbet coupling of ethanol at 613–673 K in the presence of D2 were also performed. Although there was no consequence of adding D2 to the Guerbet coupling of ethanol in terms of rate or selectivity, incorporation of deuterium into product butanol was only observed over MgO. This was attributed to the rapid exchange of H2–D2 that can occur over MgO but not over HAP. Hydrogenation of acetone occurred with ethanol as a sacrificial hydrogen donor via an MPV-like reaction whereas hydrogenation with H2 was not observed. Hydrogenation of ethene with H2 or ethanol was not observed above background. Comparing the rate of benzyl alcohol dehydrogenation to the rate of ethanol coupling over HAP and MgO suggests that the MPV-like hydrogen transfer reaction over HAP is mostly responsible for generating intermediate acetaldehyde during the Guerbet reaction instead of direct dehydrogenation.


 Please wait while we load your content...
Please wait while we load your content...