Less hindered ligands give improved catalysts for the nickel catalysed Grignard cross-coupling of aromatic ethers†
Abstract
The challenging reaction of unactivated ortho-substituted aromatic ethers with Grignard reagents has been found to be most effectively catalysed using nickel complexes of less sterically hindered ligands. Air stable, cheap, commercially available [NiCl2(PnBu3)2] stands out as an improved catalyst for this type of transformation. The improved results with this catalyst even extend to some couplings of a more activated substrate when examined at higher temperatures and at catalyst loadings down to 0.1 mol%. Unusual induction periods in these latter reactions have been related to the by-product magnesium salts acting as co-catalysts.
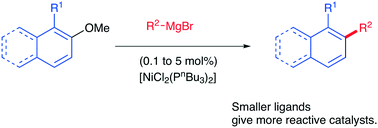


 Please wait while we load your content...
Please wait while we load your content...