Enzymatic asymmetric synthesis of chiral amino acids
Abstract
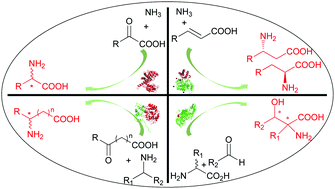
Chiral amino acids are extensively applied in the pharmaceutical, food, cosmetic, agricultural, and feedstuff industries. The development of synthetic methodologies for optically pure amino acids has been driven by their significant applications. Among the various synthesis methods for the production of chiral amino acids, enzymatic asymmetric synthesis is a unique preparation strategy that shows great potential. This review provides an overview of the reported methods for enzymatic asymmetric synthesis of chiral amino acids, including asymmetric reductive amination of keto acids, asymmetric transfer of an amino group to keto acids, enantioselective addition of ammonia to α,β-unsaturated acids, and aldol condensation of an amino acid to aldehydes.


 Please wait while we load your content...
Please wait while we load your content...