Supramolecular interaction of non-racemic benzimidazolium based ion pairs with chiral substrates†
Abstract
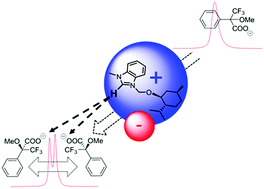
A series of novel benzimidazolium-based non-racemic ionic liquids (ILs) was synthesized from low-cost chiral terpenoid alcohols and fully characterized by the use of a wide variety of techniques, such as DSC, ESI-MS, ATR FT-IR, polarimetry as well as 1H and 13C NMR spectroscopy. The ILs were investigated as chiral shift agents for the chiral recognition of racemic mixtures of Mosher's acid potassium salt by 19F NMR spectroscopy, leading to high splitting values of the CF3 signal. Supramolecular interactions between salt and H–C2 of chiral benzimidazolium cation are responsible for the chiral recognition, as was demonstrated by experimental evidences. Indeed, the enantiomeric excess value of enantioenriched substrates depends mainly on the strength of the contact ion pairs.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...