Pinpointing the active species of the Cu(DAT) catalyzed oxygen reduction reaction†
Abstract
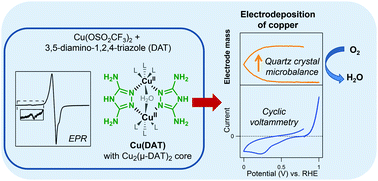
Dinuclear CuII complexes bearing two 3,5-diamino-1,2,4-triazole (DAT) ligands have gained considerable attention as a potential model system for laccase due to their low overpotential for the oxygen reduction reaction (ORR). In this study, the active species for the ORR was investigated. The water soluble dinuclear copper complex (Cu(DAT)) was obtained by mixing a 1 : 1 ratio of Cu(OTf)2 and DAT in water. The electron paramagnetic resonance (EPR) spectrum of Cu(DAT) showed a broad axial signal with a g factor of 2.16 as well as a low intensity Ms = ±2 absorption characteristic of the Cu2(μ-DAT)2 moiety. Monitoring the typical 380 nm peak with UV-Vis spectroscopy revealed that the Cu2(μ-DAT)2 core is extremely sensitive to changes in pH, copper to ligand ratios and the presence of anions. Electrochemical quartz crystal microbalance experiments displayed a large decrease in frequency below 0.5 V versus the reversible hydrogen electrode (RHE) in a Cu(DAT) solution implying the formation of deposition. Rotating ring disk electrode experiments showed that this deposition is an active ORR catalyst which reduces O2 all the way to water at pH 5. The activity increased significantly in the course of time. X-ray photoelectron spectroscopy was utilized to analyze the composition of the deposition. Significant shifts in the Cu 2p3/2 and N 1s spectra were observed with respect to Cu(DAT). After ORR catalysis at pH 5, mostly CuI and/or Cu0 species are present and the deposition corresponds to previously reported electrodepositions of copper. This leads us to conclude that the active species is of a heterogeneous nature and lacks any structural similarity with laccase.


 Please wait while we load your content...
Please wait while we load your content...