Incorporation of oxygen atoms as a mechanism for photoluminescence enhancement of chemically treated MoS2†
Abstract
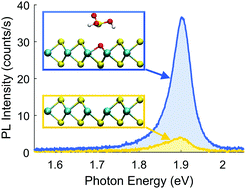
Chemical treatments to enhance photoluminescence (PL) in MoS2 have been explored extensively by experimental means in recent years. However, satisfactory theoretical explanations of the underlying mechanisms remain elusive. In this work, the surface reactions of the superacid bis(trifluoromethane)-sulfonimide (TFSI), hydrogen peroxide (H2O2), molecular oxygen (O2), and sulfuric acid (H2SO4) on a defective MoS2 monolayer have been studied using first principles calculations. An oxygen transfer reaction into a sulfur vacancy with a low activation barrier and thus significant reaction rates already at room temperature has been found. Band structure unfolding techniques show that the incorporation of oxygen atoms into sulfur vacancies restores the band structure of pristine MoS2, which is predicted to have a high PL quantum yield. PL spectroscopy is used to examine the effect of chemical treatment on PL intensity. Our experimental findings support our theoretical predictions, as PL in MoS2 is enhanced by up to a factor 20 after treatment with H2O2 or H2SO4, while the spectral shape is only slightly altered.


 Please wait while we load your content...
Please wait while we load your content...