First-principles description of oxygen self-diffusion in rutile TiO2: assessment of uncertainties due to enthalpy and entropy contributions†
Abstract
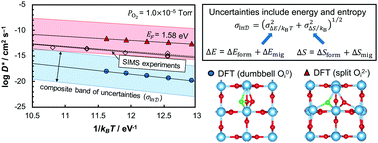
Properties related to transport such as self-diffusion coefficients are relevant to fuel cells, electrolysis cells, and chemical/gas sensors. Prediction of self-diffusion coefficients from first-principles involves precise determination of both enthalpy and entropy contributions for point defect formation and migration. We use first-principles density functional theory to estimate the self-diffusion coefficient for neutral O0i and doubly ionized Oi2− interstitial oxygen in rutile TiO2 and compare the results to prior isotope diffusion experiments. In addition to formation and migration energy, detailed estimates of formation and migration entropy incorporating both vibrational and ionization components are included. Distinct migration pathways, both based on an interstitialcy mechanism, are identified for O0i and Oi2−. These result in self-diffusion coefficients that differ by several orders of magnitude, sufficient to resolve the charge state of the diffusing species to be Oi2− in experiment. The main sources of error when comparing computed parameters to those obtained from experiment are considered, demonstrating that uncertainties due to computed defect formation and migration entropies are comparable in magnitude to those due to computed defect formation and migration energies. Even so, the composite uncertainty seems to limit the accuracy of first-principles calculations to within a factor of ±103, demonstrating that direct connections between computation and experiment are now increasingly possible.


 Please wait while we load your content...
Please wait while we load your content...
