A thermodynamic description for water, hydrogen fluoride and hydrogen dissolutions in cryolite-base molten salts
Abstract
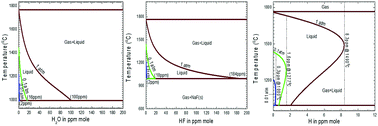
This paper presents a quantitative thermodynamic description for water, hydrogen fluoride and hydrogen dissolutions in cryolite-base molten salts, which is of technological importance to the Hall–Héroult electrolytic aluminum extraction cell. The Modified Quasichemical Model in the Quadruplet Approximation (MQMQA), as used to treat a large variety of molten salt systems, was adopted to thermodynamically describe the present liquid phase; all solid solutions were modeled using the Compound Energy Formalism (CEF); the gas phase was thermodynamically treated as an ideal mixture of all possible species. The model parameters were mainly obtained by critical evaluations and optimizations of thermodynamic and phase equilibrium data available from relative experimental measurements and theoretical predictions (first-principles calculations and empirical estimations) for the lower-order subsystems. These optimized model parameters were thereafter merged within the Kohler/Toop interpolation scheme, facilitating the prediction of gas solubility (H2O, HF and H2) in multicomponent cryolite-base molten salts using the FactSage thermochemical software. Several interesting diagrams were finally obtained in order to provide useful information for the industrial partners dedicated to the Hall–Héroult electrolytic aluminum production or other molten-salt technologies (the purification process and electroslag refining).


 Please wait while we load your content...
Please wait while we load your content...