Cocrystals of a 1,2,4-thiadiazole-based potent neuroprotector with gallic acid: solubility, thermodynamic stability relationships and formation pathways†
Abstract
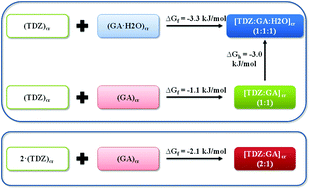
Three distinct solid forms, namely anhydrous cocrystals with 2 : 1 and 1 : 1 drug/acid ratios ([TDZ : GA] (2 : 1), [TDZ : GA] (1 : 1)), and a hydrated one having 1 : 1 : 1 drug/acid/water stoichiometry ([TDZ : GA : H2O] (1 : 1 : 1)), have been formed by cocrystallization of the biologically active 1,2,4-thiadiazole derivative (TDZ) with gallic acid (GA). The thermodynamic stability relationships between the cocrystals were rationalized in terms of Gibbs energies of the formation reactions and further verified by performing a set of competitive and exchange mechanochemical reactions. Interestingly, competitive grinding in the presence of the structurally related vanillic acid led to the formation of a new polymorphic form of the [TDZ : Vanillic acid] (1 : 1) cocrystal, which was promoted by gallic acid. The mechanochemical method was also applied to elucidate the alternative pathways of the [TDZ : GA : H2O] (1 : 1 : 1) cocrystal formation. Direct cocrystallization of TDZ with GA monohydrate was found to proceed much faster than the reaction of TDZ and anhydrous GA in the presence of an acetonitrile/water mixture, which may indicate the presence of a transitional stage. According to dissolution studies, the [TDZ : GA : H2O] (1 : 1 : 1) cocrystal was ca. 6.6 times more soluble than the parent 1,2,4-thiadiazole at pH 2.0 and 25.0 °C. The apparent two-step dehydration behavior of the [TDZ : GA : H2O] (1 : 1 : 1) cocrystal monohydrate was clarified by analyzing the intermolecular interactions of water molecules with the crystalline environment derived from solid state DFT calculations.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...