Spin crossover dynamics studies on the thermally activated molecular oxygen binding mechanism on a model copper complex†
Abstract
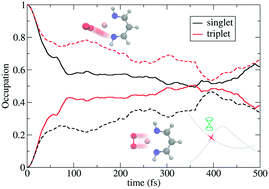
The theoretical description of the primary dioxygen (O2) binding and activation step in many copper or iron enzymes, suffers from the intrinsically electronic non-adiabaticity of the spin flip events of the triplet dioxygen molecule (3O2), mediated by spin–orbit couplings. In this work, we presented the early-stage ultrafast spin flip dynamics of O2 binding for a simplified monocopper complex, involving the coupled singlet and triplet electronic states. The on-the-fly trajectory surface hopping (TSH) simulations have identified the dynamical effects that may influence the mode of O2 coordination (end-on vs. side-on), and the electronic structures can be viewed as complexes of molecular O2 with Cu(I) or as Cu(II)-superoxide compounds. In addition, significant spin flip events are obversed within the sub-picosecond regime. We hope this work may provide complimentary insights on the traditional interpretation of O2 binding on copper complexes and subsequent catalytic reaction mechanisms.


 Please wait while we load your content...
Please wait while we load your content...