Effect of reduced graphene oxide on the solvent transport characteristics and sorption kinetics of fluoroelastomer nanocomposites†
Abstract
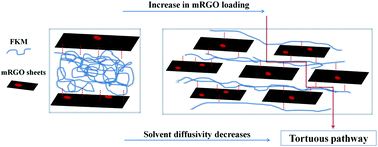
The chemical resistivity of a fluoroelastomer forms the basis of its applicability in chemical industries as gloves and inner linings of storage tanks; in automotive and aircraft industries as linings of fuel hoses, etc. In this context, the study of the molecular transport of industrially important chemicals is of considerable interest. The present study depicts the solvent transport characteristics of fluoroelastomer/reduced graphene oxide nanocomposites (FKM/mRGO) in a homologous series of aromatic hydrocarbons. The solvent diffusion characteristics of the nanocomposites were analyzed in terms of mRGO loading, nature of solvents used, temperature etc. The parameters such as equilibrium solvent uptake, diffusivity, sorptivity, and permeability were analyzed and found to decrease with an increase in filler content. This is mainly attributed to the decrease in free volume with increasing concentration of mRGO due to the improved filler–elastomer interactions that hinder the solvent transport. Swelling parameters such as swelling coefficient and swelling index were analyzed to study the polymer–filler interactions. Diffusion studies with respect to temperatures were used to estimate the enthalpy, entropy and free energy change of the diffusion process. The molecular mass between crosslinks and hence the crosslink densities were calculated by using the experimental data and the reinforcement effect of mRGO was confirmed by the decrease in Mc values and increase in crosslink densities. Theoretical models, namely phanton and affine, were compared with the experimental values, which showed a moderate agreement with affine network models.


 Please wait while we load your content...
Please wait while we load your content...