Successive C1–C2 bond cleavage: the mechanism of vanadium(v)-catalyzed aerobic oxidation of d-glucose to formic acid in aqueous solution†
Abstract
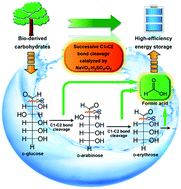
Vanadium(V)-catalyzed aerobic oxidation in aqueous solution shows high selectivity in the field of C–C bond cleavage of carbohydrates for chemicals with less carbon atoms. However, the pathway of C–C bond cleavage from carbohydrates and the conversion mechanism are unclear. In this work, we studied the pathway and the mechanism of D-glucose oxidation to formic acid (FA) in NaVO3–H2SO4 aqueous solution using isotope-labeled glucoses as substrates. D-Glucose is first transformed to FA and D-arabinose via C1–C2 bond cleavage. D-Arabinose undergoes similar C1–C2 bond cleavage to form FA and the corresponding D-erythrose, which can be further degraded by C1–C2 bond cleavage. Dimerization and aldol condensation between carbohydrates can also proceed to make the reaction a much more complicated mixture. However, the fundamental reaction, C1–C2 bond cleavage, can drive all the intermediates to form the common product FA. Based on the detected intermediates, isotope-labelling experiments, the kinetic isotope effect study and kinetic analysis, this mechanism is proposed. D-Glucose first reacts with a vanadium(V) species to form a five-membered-ring complex. Then, electron transfer occurs and the C1–C2 bond weakens, followed by C1–C2 bond cleavage (with no C–H bond cleavage), to generate the H3COO˙–vanadium(IV) complex and D-arabinose. FA is generated from H3COO˙ that is oxidized by another vanadium(V) species. The reduced vanadium species is oxidized by O2 to regenerate to its oxidation state. This finding will provide a deeper insight into the process of C–C bond cleavage of carbohydrates for chemical synthesis and provide guidance for screening and synthesizing new highly-efficient catalyst systems for FA production.


 Please wait while we load your content...
Please wait while we load your content...