Size and shape dependency of the surface energy of metallic nanoparticles: unifying the atomic and thermodynamic approaches†
Abstract
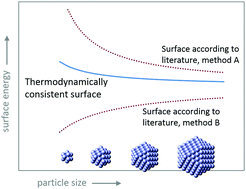
Surface energy is a fundamental property of metallic nanoparticles (MeNPs), which plays a crucial role in nucleation and growth and has strong implications for the application and environmental impact of MeNPs. Surface energy (J m−2) can be size dependent, but experimental data on surface energy trends for MeNPs are inconclusive. Computational chemistry may resolve the issue, but the location and area of the surface used for scaling, which dramatically influences the outcome and interpretation, has not been properly investigated. The size dependency of the surface energy can only be determined by scaling to the thermodynamic surface of tension. To identify this, we have derived a generalized Tolman approach for non-spherical particles, which is used to analyze the thermodynamic consistency of various surface definitions. Only the physical surface, defined here, is consistent with the surface of tension. Scaling of recent computational data for faceted MeNPs to this surface yields a low size dependency of surface energy, in good agreement with the Tolman lengths corresponding to its interfacial position. We find Tolman lengths of −0.03 nm for icosahedra and −0.04 nm for cuboctahedra of gold or silver. With this result, our approach can be used to quantify the twinning energy for icosahedral nanoparticles, being ∼0.06 J per m2 twin area. To understand the unorthodox negative Tolman lengths, we have analyzed the surface energetics of the solid–gas interface of metals in relation to the liquid–vapor interface of water. Surface entropy is found to be imperative in determining the size dependence of surface free energy. At room temperature, the influence of surface entropy on surface enthalpy is much smaller for metals than for water. It explains why these two interfaces have opposite size dependencies of the surface Gibbs free energy and opposite signs of the Tolman length. For water, forming nanodroplets or nanobubbles, the Tolman length is negative (∼−0.014 nm) for the surface enthalpy, but positive (∼+0.06 ± 0.02 nm) for the surface Gibbs free energy. For MeNPs at room temperature, both entities are negative, but at high temperature, the increased surface entropy term may cause the size dependency of surface Gibbs free energy to become reversed.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...