Probing the electric double-layer capacitance in a Keggin-type polyoxometalate ionic liquid gated graphene transistor†
Abstract
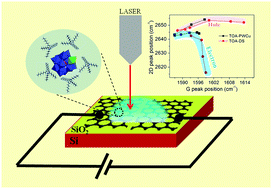
A variety of device applications has been proposed using polyoxometalate-based ionic liquids. However, the assembly of large polyoxometalate ions on surfaces and the associated interfacial properties are not well understood, particularly since the assembly is influenced by steric effects and stronger ion–ion interactions. In this study, graphene transistors gated with a polyoxometalate-based ionic liquid were probed with in situ Raman spectroscopy and charge transport studies. The ionic liquid comprised Cu-substituted lacunary Keggin anions, [PW11O39Cu]5−, which were surrounded by tetraoctyl ammonium cations, (C32H68N)+. The application of gate voltage caused these ions to assemble at the interface with graphene, which resulted in a shift of the Fermi level of the graphene monolayer grown on a copper foil. The shift was determined by the quantum capacitance, Cq, of graphene in series with the electric-double layer capacitance. Estimates of the electric-double layer thickness, spatial density of the ions and temporal rate of the assembly of the electric double-layer were obtained. This study provides insights into the microscopic understanding of the electric double-layer formation at the graphene interface.


 Please wait while we load your content...
Please wait while we load your content...