How big is the substituent dependence of the solar photolysis rate of Criegee intermediates?†
Abstract
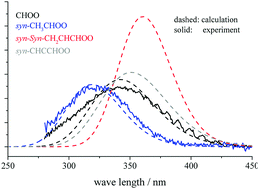
Criegee intermediates (CIs) can actively oxidize trace gases in the troposphere, and it is important to quantify their solar photolysis rates. However, experimental measurement has been challenging, and there are differences even in the UV spectra of the simplest CH2OO. In this study, we calculated the absolute UV cross sections for C1 to C3 CIs with multireference quantum chemistry and quantum dynamics methods. Our results gave peak positions, cross sections and spectral widths reproducing the experimental results for CH2OO and (CH3)2COO. For vinyl-CIs, the peak position is greatly redshifted compared to CH2OO, and the cross section is three times larger. This knowledge should help in the future detection of CIs with vinyl groups. Lastly, we showed that for C1 to C3 CIs the solar photolysis rate only varies between 0.08 and 1.03 s−1. This small substituent dependence is very different from other CI decay pathways, such as thermal decomposition and reaction with water vapor, which varied by three orders of magnitude. These rates are too slow to compete with other atmospheric decay pathways such as CI thermal decomposition or CI reaction with water vapor.


 Please wait while we load your content...
Please wait while we load your content...