Spectroscopic characterization of lithium thiophosphates by XPS and XAS – a model to help monitor interfacial reactions in all-solid-state batteries†
Abstract
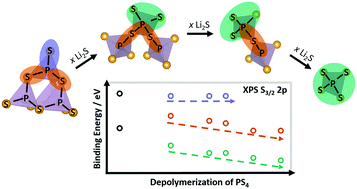
Inspired by reports of redox active interphases in all-solid-state batteries employing fast conducting lithium thiophosphate solid-state electrolytes, we investigated the compositional depolymerization of interconnected PS4 tetrahedra in (Li2S)x(P2S5)100−x glasses (50 < x < 80) by X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS). Based on the observed energy shifts with composition, we present a structural model of the three different bonding types describing the structures of either crystalline or amorphous thiophosphates. This model and reference data characterizes amorphous thiophosphates based on their inter-tetrahedral connectivity and helps to distinguish malign decomposition reactions from reversible redox reactions at the cathode active material/solid-state electrolyte interface. This work highlights the importance of a combined analytical approach and appropriate reference compounds to elucidate the interface reactions in all-solid-state battery systems.


 Please wait while we load your content...
Please wait while we load your content...