Confinement boosts CO oxidation on an Ni atom embedded inside boron nitride nanotubes†
Abstract
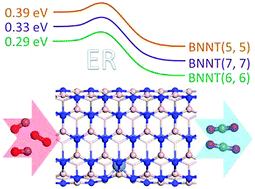
To date, most studies of heterogeneous catalysis have focused on metal particles supported on the surface of substrates. However, studies of the catalytic properties of metallic nanoparticles supported on the interior surface of nanotubes are rare. Using first-principles calculations based on density functional theory, we have studied the CO oxidation on a single nickel atom confined in a nitrogen vacancy on the inside surface of boron nitride nanotubes (BNNT). By exploring the Eley–Rideal mechanism, we find that an Ni atom embedded on the interior surface of BNNTs exhibits a much higher catalytic activity for CO oxidation when compared with Ni doped on their outside surface. In addition, the energy barriers of the rate-determining step for CO oxidation on Ni embedded on the inside wall of BNNT(5,5), BNNT(6,6) and BNNT(7,7) are 0.39, 0.29 and 0.33 eV, respectively. The results illustrate the merit of confinement for CO oxidation.


 Please wait while we load your content...
Please wait while we load your content...
