Ab initio identification of the Li-rich phase in LiFePO4†
Abstract
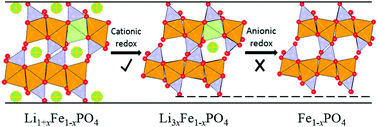
A recent discovery of anionic redox activity in Li-rich layered compounds opens a new direction for the design of high-capacity cathode materials for lithium-ion batteries. Here using extensive ab initio calculations, the thermodynamic existence of the Li-rich phase in LiFePO4 to form Li1+xFe1-xPO4 with x not exceeding 12.5% has been proved. Anionic redox activity and structural stability during delithiation are further investigated. Interestingly, it is found that Li1+xFe1−xPO4 cannot be delithiated completely and thus cannot achieve extra capacity by anionic redox activity, because the local oxygen-ion redox will cause the fracture of the rigid framework formed by phosphate tetrahedral polyanions. Although an extra capacity cannot be realized, the excess Li-ions at Fe sites can enhance the Li-ion diffusivity along the adjacent [010] channel and contribute to the shift from 1D to 2D/3D diffusion. This study provides a fresh perspective on olivine-type LiFePO4 and offers some important clues on designing Li-rich cathode materials with high energy density.


 Please wait while we load your content...
Please wait while we load your content...