Tuning electronic and magnetic properties of Mn-mullite oxide sub-nanoclusters via MnOn polyhedron units†
Abstract
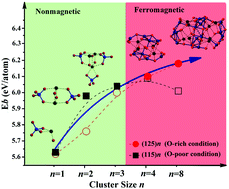
Ternary oxide nano-clusters compared to unary metallic and binary ones potentially exhibit more remarkable properties due to their higher stoichiometric flexibility in addition to cluster size variations. Herein, by combining with the structural searching scheme CALYPSO, we have built a series of Mn-mullite oxide clusters (SmxMnyOz)n {(xyz) = (125); (115); n = 1–4, 8} prior to investigation of their geometric and electronic structures via first-principles calculations. In small size regime (n < 4), (SmxMnyOz)n prefer nonstoichiometric (Sm1Mn1O5)n phases composed of nonmagnetic MnO4 tetrahedrons. When n ≧ 4, the clusters tend to develop as stoichiometric (Sm1Mn2O5)n species, including magnetic MnOn polyhedrons and Mn–Mn dimers, which contribute 3d-orbitals (dz2 and/or dx2−y2) around the Fermi levels. The different magnetic behaviors of nonstoichiometric and stoichiometric species originate from the distinct couplings of MnOn polyhedronal units, wherein Mn atoms experience different ligand fields and thus display different spin states. Such findings enable the tuning of electronic properties and potential applications in heterogeneous catalysis, electrochemical catalysis, and the related fields via engineering cluster size and stoichiometry.


 Please wait while we load your content...
Please wait while we load your content...