On the structure of superbasic (MgO)n sites solvated in a faujasite zeolite†
Abstract
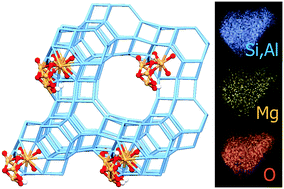
We report the synthesis and characterisation of a HY/MgO zeolite/oxide nanocomposite material with high crystallinity and highly dispersed, highly basic MgO sites. Preparation was optimized in order to preserve sample crystallinity, to avoid the formation of mesoporosity and to minimize the formation of separate Mg-containing phases. These features were checked by means of electron microscopy, X-ray powder diffraction, porosimetry and IR spectroscopy. A highly dispersed material was obtained, comprising nanoclusters of magnesium oxide and hydroxide hosted by the microporous zeolite framework. The location and structure of the Mg-containing clusters have been studied by means of a combination of Rietveld refinement of XRPD data and high quality quantum mechanical simulations. The refinement has shown the presence of magnesium and oxygen atoms in the double six-membered ring cages, consistent with the presence of mononuclear Mg moieties. However, the composition and IR spectroscopy demonstrate that other Mg species must exist, likely located in the zeolite pores. In order to propose candidate structures for these species, several hypothetic periodic models of the material were built by placing (MgO)n clusters in different locations of the zeolite structure, taking into account the material composition and other constraints imposed by the experimental observations. Periodic structures with P1 symmetry were optimized at the B3LYP-D*/DZVP level with the CRYSTAL code and classified according to their stability. Two families of possible sites were identified: highly solvated (MgO)n units in narrow cavities and less coordinated clusters in the supercages. The stability of these clusters appears to be regulated by the ability of Mg2+ and O2− ions to interact with the pore walls and by the formation of Mg–OH species as result of the reaction of Mg–O couples with remaining acidic protons. The reactivity of four representative models with CO2 has been simulated at the B3LYP-D*/TZVP level. CO2 forms very stable linear end-on adducts with low coordinated Mg ions in most cases. Isolated sites give rise to bridge bidentate complexes in agreement with previous spectroscopic observations. The formation of hydrogen-carbonates is observed only on specific sites, through a process having a low adsorption energy because of the high deformation of the adsorption site.


 Please wait while we load your content...
Please wait while we load your content...