Acemetacin–phosphatidylcholine interactions are determined by the drug ionization state†
Abstract
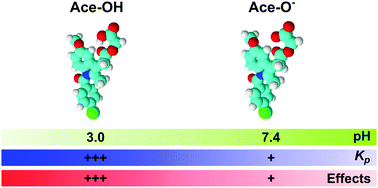
Gastrointestinal (GI) toxicity is a major drawback of the chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs). The NSAIDs topical actions on the protective phospholipid layers of the GI mucosa seem to be a central toxicity mechanism of these pharmaceuticals. This work describes the interactions of acemetacin, a commercialized NSAID, with 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) bilayers at pH 3.0, 5.0, and 7.4. This pH range was chosen to mimic the pH gradient found in the gastric mucosa, and to ultimately gain insights into the mechanisms underlying the acemetacin-induced gastric toxicity. Various experimental techniques were combined to characterize the partitioning of acemetacin in DMPC bilayers, and its effects on the phase transition behavior, as well as the structure and dynamics of DMPC bilayers. The acemetacin–DMPC interactions were clearly pH-dependent. The neutral (protonated) form of acemetacin had more affinity for the DMPC bilayer than the negatively charged form. Due to the higher affinity of neutral acemetacin, the drug effects on the phase transition and the structure and dynamics of the DMPC bilayer were more pronounced at lower pH values. In general, acemetacin decreased the temperature and the cooperativity of the lipid phase transition and induced changes in the packing and dynamics of the DMPC bilayer. These results support the hypothesis that acemetacin-induced gastric toxicity may be related to its effects on the protective phospholipid layers of the mucosal barrier.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...