Molecular insights into avibactam mediated class C β-lactamase inhibition: competition between reverse acylation and hydrolysis through desulfation†
Abstract
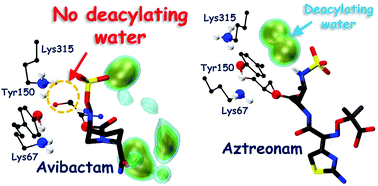
Avibactam is one of the promising next generation β-lactamase inhibitors due to its exceptional inhibition against wide-spectrum serine β-lactamases. The unusual reversible acylation mechanism has particularly gained interest to explain the inhibition mechanism of avibactam. We explore the mechanism of acylation and deacylation involving avibactam in class-C β-lactamases (CBLs) through hybrid quantum mechanical/molecular mechanical (QM/MM) enhanced sampling molecular dynamics (MD) simulations. Based on these computations, we probe the kinetic stability of the acyl–enzyme complex formed by avibactam and CBLs, thereby gaining molecular level insights into the avibactam-mediated inhibition of CBLs.


 Please wait while we load your content...
Please wait while we load your content...