Competition between salt bridge and non-zwitterionic structures in deprotonated amino acid dimers†
Abstract
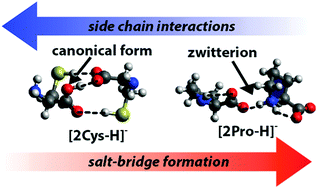
Structures of deprotonated Cys, Asp, Glu, Phe, Pro, His homo dimers as well as [2Cys − 3H]−, [Asp + Glu − H]− and [2Glu − 2H + Na]− are investigated with infrared multiple-photon dissociation (IRMPD) spectroscopy between 650 and 1850 cm−1 and theory. The IRMPD spectra of all investigated complexes but [2His − H]−, [2Phe − H]− and [2Pro − H]− indicate that the structures consist of a neutral non-zwitterionic (NZ) and a deprotonated form of the amino acids. In contrast, the spectrum of [2His − H]− is complex and indicates the presence of multiple isomers and/or interactions between His and [His − H]−, so that its structure differs from that of the other deprotonated amino acid dimers. For [2Phe − H]− and especially for [2Pro − H]−, some IRMPD bands can only be explained by the presence of salt bridge (SB) structures in the dimer in which a deprotonated amino acid interacts with a zwitterionic neutral amino acid. Computational results indicate that SB structures are lower in energy at 298 K than corresponding NZ structures for neutral-anion complexes in which SB formation is not disrupted by amino acid side chains or conformational constraints, such as in [2Glu − H]− and [2Cys − 3H]− for which NZ structures are most consistent with experimental results. For deprotonated amino acid dimers in which these interfering interactions are absent, such as in [2Phe − H]− and [2Pro − H]−, the higher number of hydrogen bonds in SB compared to NZ structures stabilize the formation of zwitterionic neutral amino acids and consequently SB structures in agreement with results from IRMPD spectroscopy. These results suggest that SB structures likely occur in deprotonated peptide or protein ions at hydrophobic sites, such as protein–protein interfaces or in the interior of proteins, where interfering functional groups will not disrupt SB formation.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
