Electron-stimulated reactions in nanoscale water films adsorbed on α-Al2O3(0001)†
Abstract
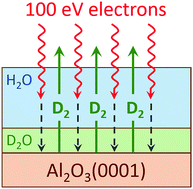
The radiation-induced decomposition and desorption of nanoscale amorphous solid water (D2O) films adsorbed on an α-Al2O3(0001) surface was studied at low temperature in ultrahigh vacuum using temperature programmed desorption (TPD) and electron stimulated desorption (ESD) with a mono-energetic, low energy electron source. ESD yields of molecular products (D2, O2 and D2O) and the total sputtering yield increased with increasing D2O coverage up to ∼15 water monolayers (i.e. ∼15 × 1015 cm−2) to a coverage-independent level for thicker water films. Experiments with isotopically-layered water films (D2O and H2O) demonstrated that the highest water decomposition yields occurred at the interfaces of the nanoscale water films with the alumina substrate and vacuum. However, the increased reactivity of the water/alumina interface is relatively small compared to the enhancements in the non-thermal reactions previously observed at the water/Pt(111) and water/TiO2(110) interfaces. We propose that the relatively low activity of Al2O3(0001) for the radiation-induced production of molecular hydrogen is associated with lower reactivity of this surface with hydrogen atoms, which are likely precursors for the formation of molecular hydrogen.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
