Theoretical and experimental investigations of the electronic/ionic conductivity and deprotonation of Ni3−xCoxAl-LDHs in an electrochemical energy storage system†
Abstract
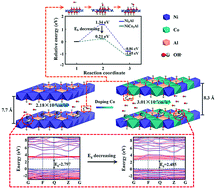
The remarkable effect of divalent transition metal ions on the electrochemical performance of transition metal-based layered double hydroxides (LDHs) was systematically investigated via computational and experimental approaches. Ni3−xCoxAl-LDHs (x = 0, 1, 2, and 3) were synthesized on carbon paper by a unipolar pulse electrodeposition (UPED) method and used as electrodes in energy storage systems. The structures were characterized by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). Their electrochemical performance was evaluated by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The mechanism of different electrochemical performances with various divalent transition metal ions was investigated by the density functional theory (DFT) plus U method and molecular dynamics (MD) simulations. The computational and experimental data demonstrated that the electronic and ionic conductivity and deprotonation of NiAl-LDHs were improved by doping Co species, and the incorporation of Co and Ni cations enabled LDHs to exhibit a larger interlayer spacing which can facilitate the diffusion of OH− ions, indicating that NiCo2Al-LDHs had the highest specific capacitance.


 Please wait while we load your content...
Please wait while we load your content...