Anisotropic chemical strain in cubic ceria due to oxygen-vacancy-induced elastic dipoles†
Abstract
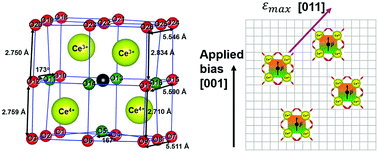
Accurate characterization of chemical strain is required to study a broad range of chemical–mechanical coupling phenomena. One of the most studied mechano-chemically active oxides, nonstoichiometric ceria (CeO2−δ), has only been described by a scalar chemical strain assuming isotropic deformation. However, combined density functional theory (DFT) calculations and elastic dipole tensor theory reveal that both the short-range bond distortions surrounding an oxygen-vacancy and the long-range chemical strain are anisotropic in cubic CeO2−δ. The origin of this anisotropy is the charge disproportionation between the four cerium atoms around each oxygen-vacancy (two become Ce3+ and two become Ce4+) when a neutral oxygen-vacancy is formed. Around the oxygen-vacancy, six of the Ce3+–O bonds elongate, one of the Ce3+–O bond shorten, and all seven of the Ce4+–O bonds shorten. Further, the average and maximum chemical strain values obtained through tensor analysis successfully bound the various experimental data. Lastly, the anisotropic, oxygen-vacancy-elastic-dipole induced chemical strain is polarizable, which provides a physical model for the giant electrostriction recently discovered in doped and non-doped CeO2−δ. Together, this work highlights the need to consider anisotropic tensors when calculating the chemical strain induced by dilute point defects in all materials, regardless of their symmetry.


 Please wait while we load your content...
Please wait while we load your content...
