Newly proposed proton-abstraction roundabout with backside attack mechanism for the SN2 reaction at the nitrogen center in F− + NH2Cl†
Abstract
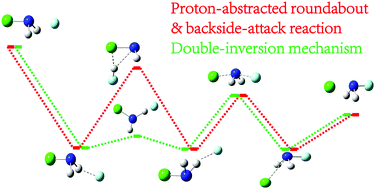
Recent studies have improved our understanding of the mechanism and dynamics of the bimolecular nucleophilic substitution (SN2) reaction at the carbon center. Nonetheless, the SN2 reaction at the nitrogen center has received scarce attention and is less understood. Herein, we propose a new reaction mechanism for the SN2 reaction at the nitrogen center in the F− + NH2Cl reaction using ab initio molecular dynamics calculations. The newly proposed mechanism involves the rotation of NHCl with one proton of NH2Cl abstracted by the nucleophile, followed by the classical backside-attack process. The double-inversion mechanism revealed recently for the SN2 reaction at the carbon center is also observed for the title reaction at the nitrogen center. In contrast to the F− + CH3Cl reaction with a proton abstraction-induced first inversion transition state, the F− + NH2Cl reaction is a hydrogen bond-induced inversion. This newly proposed reaction mechanism opens a reaction channel to avoid the proton abstraction mechanism at low collision energy. The double-inversion mechanism of the title reaction with a negative first-inversion transition relative to the energy of the reactants is expected to have larger contribution to the reaction rate than the F− + CH3Cl reaction with a positive first-inversion transition state.


 Please wait while we load your content...
Please wait while we load your content...