Osteocalcin facilitates calcium phosphate ion complex growth as revealed by free energy calculation†
Abstract
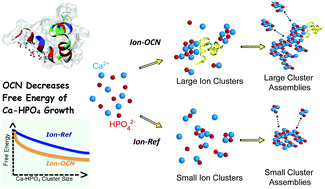
The nanoscopic structural and thermodynamic basis of biomolecule-regulated assembly and crystallization of inorganic solids have a tremendous impact on the rational design of novel functional nanomaterials, but are concealed by many difficulties in molecular-level characterization. Here we demonstrate that the free energy calculation approach, enabled by combining advanced molecular simulation techniques, can unravel the structural and energetic mechanisms of protein-mediated inorganic solid nucleation. It is observed that osteocalcin (OCN), an important non-collagenous protein involved in regulating bone formation, promotes the growth of nanosized calcium phosphate (CaP) ion clusters from a supersaturated solution. Free energy calculation by umbrella sampling indicates that this effect by OCN is prominent at the scale of 1 to 3 nm ion-association complexes (IACs). The binding interactions between gamma-carboxyl glutamate and the C-terminal and, interestingly, the arginine side chains of OCN and IACs stabilize under-coordinated IACs, thus promoting their growth. The promoter effect of OCN on the enlargement and further aggregation of IACs into cluster assemblies of tens of nm are confirmed by conventional molecular dynamics simulation and dynamic light scattering experiments. To the best of our knowledge, this is the first time that the free energy landscape of the early stages of CaP nucleation is shown. The free energy change as a function of IAC size shares the feature of decreasing monotonically as shown previously for the calcium carbonate system. Therefore, the nucleation of both these major biominerals apparently involves an initial phase of liquid-like ionic aggregates. The structural and thermodynamic information regarding OCN–CaP interactions amplifies the current understanding of biomineralization mechanisms at the nanoscale, with general relevance to biomolecule-tuned fabrication of inorganic materials.


 Please wait while we load your content...
Please wait while we load your content...