Thermal stability of dialkylimidazolium tetrafluoroborate and hexafluorophosphate ionic liquids: ex situ bulk heating to complement in situ mass spectrometry†
Abstract
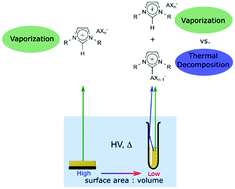
Thermal decomposition (TD) products of the ionic liquids (ILs) [CnC1Im][BF4] and [CnC1Im][PF6] ([CnC1Im]+ = 1-alkyl-3-methylimidazolium, [BF4]− = tetrafluoroborate, and [PF6]− = hexafluorophosphate) were prepared, ex situ, by bulk heating experiments in a bespoke setup. The respective products, CnC1(C3N2H2)BF3 and CnC1(C3N2H2)PF5 (1-alkyl-3-methylimidazolium-2-trifluoroborate and 1-alkyl-3-methylimidazolium-2-pentafluorophosphate), were then vaporized and analyzed by direct insertion mass spectrometry (DIMS) in order to identify their characteristic MS signals. During IL DIMS experiments we were subsequently able, in situ, to identify and monitor signals due to both IL vaporization and IL thermal decomposition. These decomposition products have not been observed in situ during previous analytical vaporization studies of similar ILs. The ex situ preparation of TD products is therefore perfectly complimentary to in situ thermal stability measurements. Experimental parameters such as sample surface area to volume ratios are consequently very important for ILs that show competitive vaporization and thermal decomposition. We have explained these experimental factors in terms of Langmuir evaporation and Knudsen effusion-like conditions, allowing us to draw together observations from previous studies to make sense of the literature on IL thermal stability. Hence, the design of experimental setups are crucial and previously overlooked experimental factors.


 Please wait while we load your content...
Please wait while we load your content...