Theoretical investigation of M@Pb122− and M@Sn122− Zintl clusters (M = Lrn+, Lun+, La3+, Ac3+ and n = 0, 1, 2, 3)†
Abstract
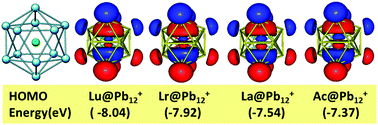
The positions of lawrencium (Lr), lutetium (Lu), actinium (Ac) and lanthanum (La) in the periodic table have been a controversial topic for quite some time. According to studies carried out by different groups with their justifications, these elements may potentially be placed in the d-block, p-block or all four in a 15 element f-block. The present work looks into this issue from a new perspective, which involves encapsulation of these four elements into Zintl ion clusters, Pb122− and Sn122−, followed by the determination of the structural, thermodynamic and electronic properties of these endohedral M@Pb122− and M@Sn122− clusters (M = Lrn+, Lun+ with n = 0, 1, 2, 3) using first principles based density functional theory (DFT). These parameters are compared with similar clusters encapsulated La3+ and Ac3+ ions in order to seek out similarities and differences to draw conclusions about their placement in the periodic table. For the first time the structural, energetic, and electronic properties of these metal atom/ion encapsulated Pb122− and Sn122− clusters have been investigated thoroughly. Structural parameters such as bond distances, geometry and symmetry, electronic properties viz. the density of states, the molecular orbital ordering, the electron localization function, bond critical point properties and charge distributions have been analyzed. Additionally, the thermodynamic property of the binding energy during the encapsulation process has also been calculated. All M@Pb12+ and M@Sn12+ (M = Lr and Lu) clusters form stable 18 bonding electron magic number systems with shell closing. They show negative values of binding energy and relatively large HOMO–LUMO energy gaps indicating the stability of such clusters. All the calculated parameters for Lr encapsulated clusters closely match with the corresponding calculated parameters of Lu encapsulated clusters, confirming the similarity between Lr and Lu metal atoms in various oxidation states, though their atomic ground state valence electronic configurations are different. The effect of spin orbit coupling has also been investigated using the ZORA approach. It is interesting to discover that La and Ac showed striking similarities to Lr and Lu with respect to all the properties investigated and have formed a stable 18-electron system.


 Please wait while we load your content...
Please wait while we load your content...