Ultra-low friction mechanism of highly sp3-hybridized amorphous carbon controlled by interfacial molecule adsorption†
Abstract
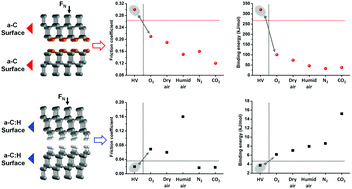
The friction behaviors of highly sp3-hybridized carbon films, including ultra-nanocrystalline diamond and diamond-like carbon materials, strongly depend on the atmosphere. However, the roles of the corresponding molecules in the interfacial bonding characteristics remain a subject of debate. By means of density functional theory calculations, this study aims to fill a knowledge gap about the correlation between the evolving contact quality induced by the adsorption of molecules, and the friction behavior of highly sp3-bonded carbons. The results prove that gas–solid adsorption is responsible for the diversity in friction coefficients of ultra-nanocrystalline diamond and diamond-like carbons in different atmospheres. This study emphasizes the role of terminal states in friction coefficients, and demonstrates that electron lubrication is another available strategy for hydrogenated diamond-like carbons to achieve ultra-low friction. This conclusion is validated by the ultra-low friction coefficient (∼0.009) of hydrogenated diamond-like carbons in a dry nitrogen atmosphere. These findings provide atomic scale descriptions of the surface passivation mechanisms for ultra-nanocrystalline diamond and diamond-like carbons, which contribute to our understanding of their macro-scale friction behaviors.


 Please wait while we load your content...
Please wait while we load your content...