On the contribution of f electrons to the quadratic hyperpolarizability: the case of lanthanide terpyridyl complexes†
Abstract
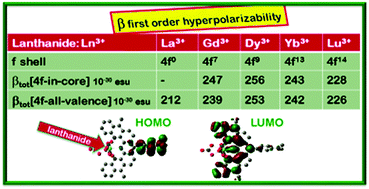
Over the last decades, trivalent lanthanide ions (Ln3+) have gained much attention due to their peculiar luminescence, which opened the way to a broad range of applications, from medical diagnostic to lasers. Their impact on nonlinear optical (NLO) properties also attracted interest, especially in the framework of lanthanide complexes. Several experimental studies demonstrated that the quadratic hyperpolarizability varies with the number of 4f-electrons, with a stronger effect on dipolar than octupolar components. The main interpretation put forward to explain the observed trends relied on the polarizable character of the 4f-electrons. We report here a first step towards understanding the role of 4f-electrons in NLO responses, considering a series of dipolar terpyridyl-trinitro lanthanide complexes LLn(NO3)3 (Ln = Gd, Dy, Yb, Lu as well as La and Y; L = terpyridil-like ligand). Using DFT and TD-DFT we investigate their linear and non-linear optical properties. Consistently with earlier experimental findings, simulated UV-visible spectra show minor changes by varying Ln. The same holds for dipole moments and polarizabilities, whereas the nature of the lanthanide affects hyperpolarizabilities. It is shown that the observed changes are not a direct effect of the 4f-electrons that behave like core electrons.


 Please wait while we load your content...
Please wait while we load your content...