Red-shifting and blue-shifting OH groups on metal oxide surfaces – towards a unified picture
Abstract
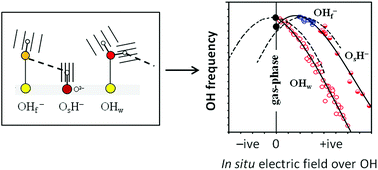
We analyse the OH vibrational signatures of 56 structurally unique water molecules and 34 structurally unique hydroxide ions in thin water films on MgO(001) and CaO(001), using DFT-generated anharmonic potential energy surfaces. We find that the OH stretching frequencies of intact water molecules on the surface are always downshifted with respect to the gas-phase species while the OH− groups are either upshifted or downshifted. Despite these differences, the main characteristics of the frequency shifts for all three types of surface OH groups (OHw, OsH and OHf) can be accounted for by one unified expression involving the in situ electric field from the surrounding environment, and the gas-phase molecular properties of the vibrating species (H2O or OH−). The origin behind the different red- and blueshift behaviour can be traced back to the fact that the molecular dipole moment of a gas-phase water molecule increases when an OH bond is stretched, but the opposite is true for the hydroxide ion. We propose that familiarity with the relations presented here will help surface scientists in the interpretation of vibrational OH spectra for thin water films on ionic crystal surfaces.


 Please wait while we load your content...
Please wait while we load your content...