Determination of accurate backbone chemical shift tensors in microcrystalline proteins by integrating MAS NMR and QM/MM†
Abstract
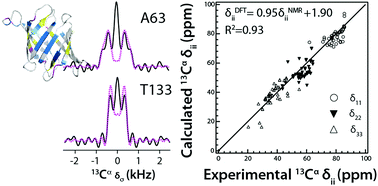
Chemical shifts are highly sensitive probes of local conformation and overall structure. Both isotropic shifts and chemical shift tensors are readily accessible from NMR experiments but their quantum mechanical calculations remain challenging. In this work, we report and compare accurately measured and calculated 15NH and 13Cα chemical shift tensors in proteins, using the microcrystalline agglutinin from Oscillatoria agardhii (OAA). Experimental 13Cα and 15NH chemical tensors were obtained by solid-state NMR spectroscopy, employing tailored recoupling sequences, and for their quantum mechanics/molecular mechanics (QM/MM) calculations different sets of functionals were evaluated. We show that 13Cα chemical shift tensors are primarily determined by backbone dihedral angles and dynamics, while 15NH tensors mainly depend on local electrostatic contributions from solvation and hydrogen bonding. In addition, the influence of including crystallographic waters, the molecular mechanics geometry optimization protocol, and the level of theory on the accuracy of the calculated chemical shift tensors is discussed. Specifically, the power of QM/MM calculations in accurately predicting the unusually upfield shifted 1HN G26 and G93 resonances is highlighted. Our integrated approach is expected to benefit structure refinement of proteins and protein assemblies.


 Please wait while we load your content...
Please wait while we load your content...
