Liquid worm-like and proto-micelles: water solubilization in amphiphile–oil solutions†
Abstract
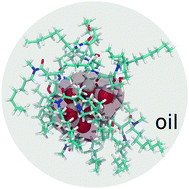
Noncovalent interactions determine the structure–property relationship of materials. Self-assembly originating from weak noncovalent interactions represents a broad variety of solution-based transformations spanning micellization and crystallization, which, nevertheless, conforms to neither colloid nor solution sciences. Here, we investigate the weak self-assembly in water–amphiphile–oil solutions to understand the connection between the amphiphilic molecular structure and water solubilization in oil. X-ray and neutron scattering, converged with large-scale atomistic molecular dynamics simulations, support the fact that the amphiphiles assemble into liquid worm-like micelles and loose inverted proto-micelles. The inverted proto-micelles are energetically ready to accommodate a higher amount of water. These structures arise from a balance of intermolecular interactions controlled by the amphiphile tail-group structures. Thus, by linking the aggregate morphology to the molecular structure, this work provides insights on the molecular design for control of water solubility and dispersion in oil.


 Please wait while we load your content...
Please wait while we load your content...
