The effect of alkyl chain length on the structure and thermodynamics of protic–aprotic ionic liquid mixtures: a molecular dynamics study†
Abstract
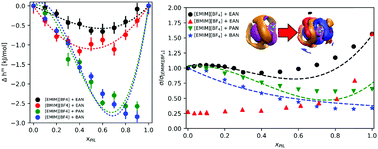
Mixtures of alkylammonium based protic ionic liquids and alkylmethylimidazolium based aprotic ionic liquids were studied by means of molecular dynamics simulations. Close to ideal mixing is observed in most studied magnitudes; however, the effect of increasing alkyl chain length in each of the cations is markedly different, with longer protic cations showing larger deviations, especially with regards to mixing enthalpy, which exhibits a strong compound forming tendency. The compound forming nature of these protic ionic liquids is shown to induce sharp changes in their local environment upon mixing.


 Please wait while we load your content...
Please wait while we load your content...